Lay Summary
PIGV-CDG, also known as hyperphosphatasia with mental retardation syndrome 1 (HPMRS1) or Mabry syndrome, is a rare inherited condition that affects many systems in the body. There are 34 cases reported to date in the medical literature. PIGV-CDG is classified as a disorder of GPI anchor biosynthesis. PIGV-CDG is caused when an individual as a mutation on both copies of their PIGV gene. The PIGV gene provides instructions for making a protein that participates in building GPI anchors, which are molecules that “anchor” certain proteins to the cell surface. The PIGV protein is involved in adding the second mannose group to the growing GPI anchor. Mutations in the PIGV gene cause defects in GPI-anchored proteins which make them unstable or unable to attach to the surface. Symptoms of PIGV-CDG being at infancy and are primarily characterized by severe developmental delay, facial and structural abnormalities, and hyperphosphatasia. PIGV-CDG is usually diagnosed through genetic testing, however testing for the presence of GPI-ancchored proteins on certain blood cells or fibroblast cells can also identify PIGV-CDG. There are no approved treatments for PIGV-CDG and treatment is focused on the management of specific symptoms and preventing complications.
Overview
Phosphatidylinositol glycan class V congenital disorder of glycosylation (PIGV-CDG) is a rare autosomal recessive genetic disorder. The first reported case of PIGV-CDG was in 2010 and 34cases have been reported in the literature to date 1–7. PIGV-CDG is commonly referred to as hyperphosphatasia with mental retardation syndrome 1 (HPMRS1) or Mabry syndrome.
The PIGV gene encodes a mannosyltransferase enzyme that is responsible for the addition of the second mannose group on the growing glycosylphosphatidylinositol (GPI) anchor protein during GPI anchor protein biosynthesis. GPI anchors are an important mechanism of attachment for proteins to the cell surface, and these proteins are known as GPI-anchored proteins. Mutations in PIGV result in defects in GPI-anchored proteins, which are critical to the development and function of many organs, especially the nervous system8,9.
Symptoms begin at infancy and the characteristic presentation includes severe global developmental delay, facial dysmorphisms, short finger bones, and hyperphosphatasia4,5,10. Hirschsprung disease is another symptom observed in some patients. Although a definitive diagnosis can only be achieved through genetic sequencing, analyzing blood cells or fibroblast cells for an absence of GPI-anchored proteins can help diagnose disorders of GPI-anchor biosynthesis7. No treatment is currently available for PIGV-CDG.
Synonyms
- Glycosylphosphatidylinositol biosynthesis defect 2
- Hypophosphatasia with mental retardation syndrome 1 (HPMRS1)
- Mabry Syndrome
- PIGV deficiency
Inheritance
PIGV-CDG is an autosomal recessive disorder, meaning an affected individual inherits one defective copy of the gene from each asymptomatic parent.
Gene Function
The PIGV gene encodes the enzyme GPI mannosyltransferase 2 (GPI-MTII). The PIGV enzyme adds the second mannose group to the growing glycosylphosphatidylinositol (GPI) anchor during GPI anchor protein biosynthesis in the endoplasmic reticulum (ER)8,9.
GPI-Anchored Protein Biosynthesis
GPI-anchored protein biosynthesis is one of the major glycosylation pathways that attach glycans to lipid molecules within cells. Many proteins are attached to the cell surface by GPI anchors, which are referred to as GPI-anchored proteins.
The core structure of GPI consists of phosphatidylinositol (PI), glucosamine (GlcN), three mannose sugars (Man3), and phosphoethanolamine (EtN-P) connected to each other in that sequence (Figure 1). GPI-anchored proteins are attached to the GPI by forming a bond between the EtN-P group of the GPI core and the C-terminus of newly synthesized proteins in the ER lumen11.
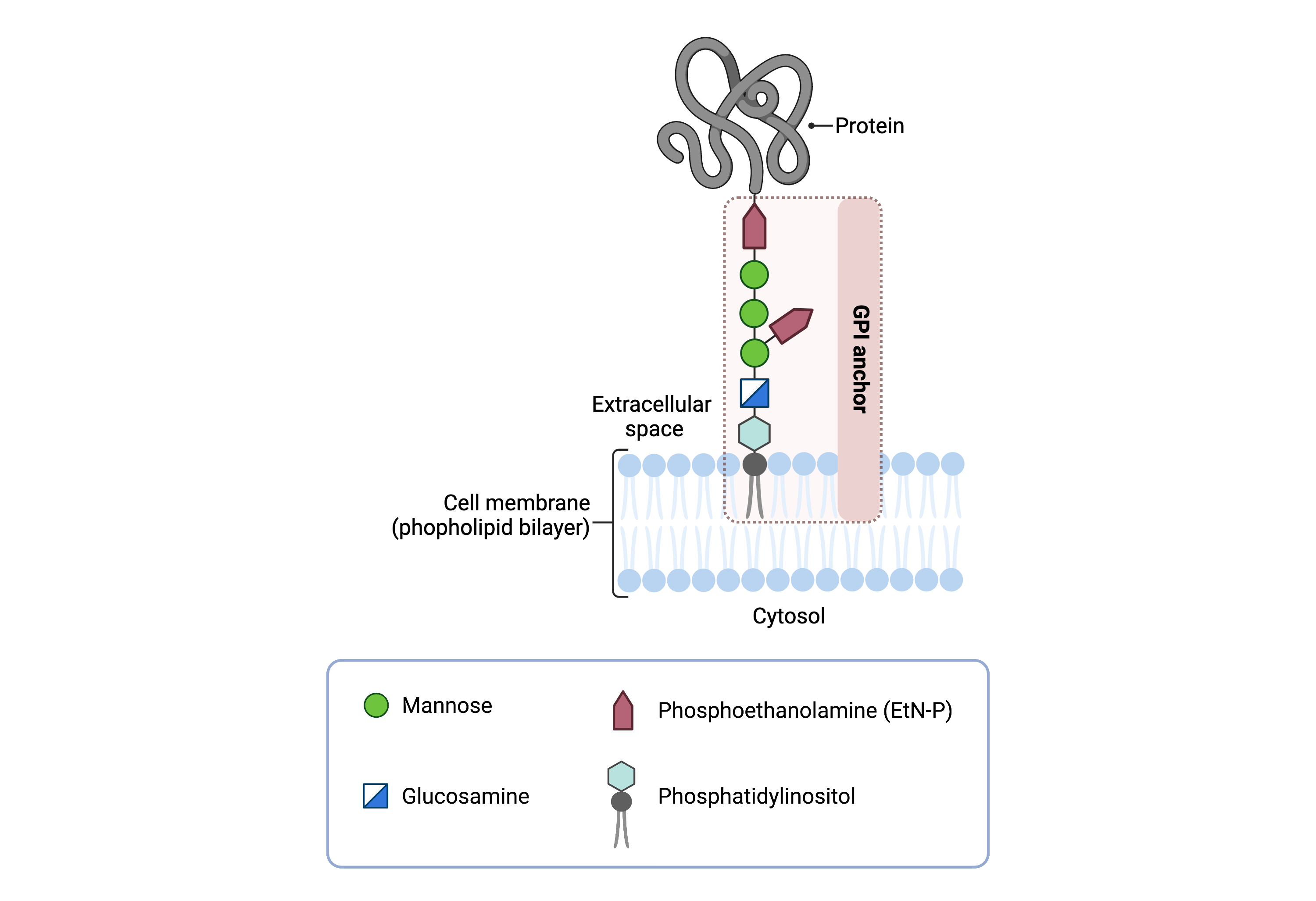
Figure 1. Overview of GPI-anchored protein structure.
The GPI anchor is added to proteins, generating GPI-anchored proteins. The GPI anchor section lodges into the membrane, attaching the protein to the membrane. The GPI anchor involves phosphatidylinositol, glucosamine, mannose and phosphoethanolamine.
The generation of GPI-anchored proteins is a multi-step process involving more than 30 enzymes and can be divided into the following steps (Figure 2) 12–14:
- GPI anchor synthesis
- Protein attachment
- Lipid/glycan remodelling and protein transport
GPI anchor synthesis and protein attachment is largely carried out by a series of enzymes encoded by the PIG genes, while enzymes encoded by the PGAP genes facilitate remodeling of the GPI-anchored protein.
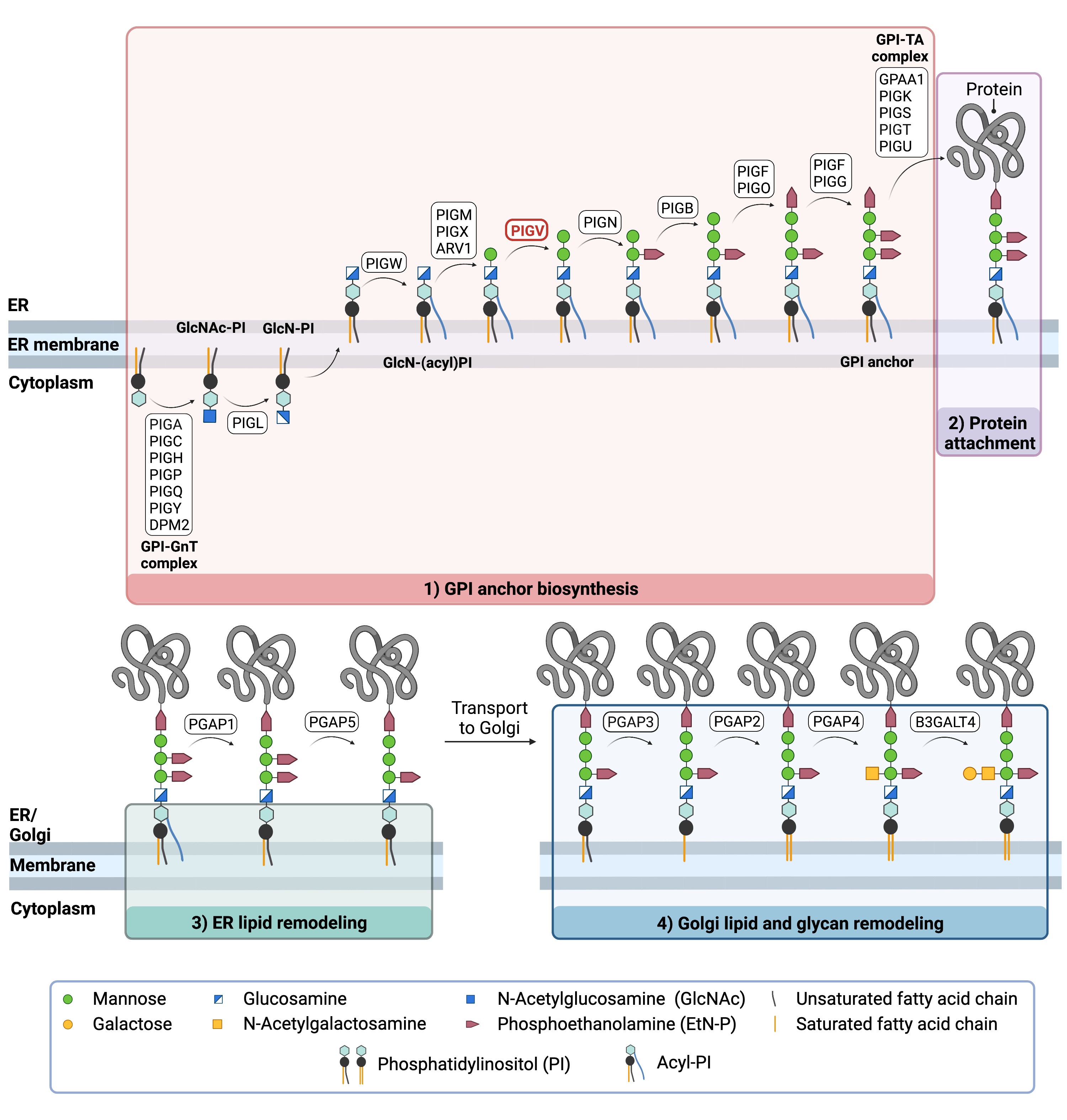
Figure 2. Overview of GPI-anchored protein biosynthesis.
GPI-anchored protein biosynthesis involves on a series of enzymatic reactions. First, the GPI anchor core is built on the ER membrane, where enzymes modify the lipid and glycan portions, generating PI-GlcN-Man3-EtN-P. The protein is then attached to the GPI anchor in the ER, before further modifications are made to the lipid and glycan portions in the ER and the Golgi. PIG-V catalyzes the attachment of the second mannose residue to the growing GPI anchor in the ER.
GPI Anchor Synthesis
The first stage of GPI anchor biosynthesis involves the stepwise construction of the GPI anchor. N-acetylglucosamine (GlcNAc) is added to the lipid phosphatidylinositol (PI), generating GlcNAc-PI. The transfer of GlcNAc is catalyzed by GPI GlcNAc transferase (GPI-GnT). The PIG-L enzyme further modifies the GPI anchor structure before it is transferred into the ER. Once in the ER, additional enzymes continue to modify the GPI anchor structure, including PIG-V which adds the second of three mannose groups to the growing GPI anchor8. Additional enzymes then further modify the GPI anchor structure, generating the GPI anchor core (PI-GlcN-Man3-EtN-P)13,15.
Protein Attachement
Once the GPI anchor has been synthesized, it is transferred en bloc to a protein with a C-terminal GPI attachment signal sequence by GPI transamidase (GPI-TA). The five-protein enzyme complex catalyzes the simultaneous cleavage of the signal sequence and attachment of the GPI anchor to the newly synthesized protein12.
Lipid/Glycan Remodeling and Protein Transport
After the protein has been attached to the GPI anchor, both the glycan and lipid portion of the anchor undergo modifications (referred to as remodeling) in the ER and Golgi by post-GPI attachment to protein (PGAP) enzymes or the glycosyltransferase B3GALT4. The complete GPI-anchored protein is then transported to the plasma membrane where it associates with other GPI-anchored proteins in lipid rafts16.
Disease Mechanism
Mutations in the PIGV gene decrease the stability of the protein or reduce enzyme function, leading to a reduction in functional GPI-anchored protein at the cell surface7. GPI-anchored proteins have several critical functions in the cell such as adhesion molecules, receptors, and enzymes in signal transduction pathways. GPI-anchored proteins are known to be expressed during neurogenesis, and even a slight deficiency in GPI-anchored proteins results in defects in neuronal development 17.
Additionally, hyperphosphatasia results from secretion of alkaline phosphatase (ALP). It was found that ALP secretion requires GPI transamidase, which recognizes incomplete GPIs and cleaves a hydrophobic peptide signal, causing secretion of ALP18,19.
Mutations
The PIGV gene is located on Chromosome 1 (1p36.11). The most common mutation reported is c.1022C>A (p.Ala341Val), which has been found as a homozygous and heterozygous mutation. Severe mutations, such as c.905T>C (p.Leu302Pro), present as multiple congenital malformation syndrome4.
Signs and Symptoms
- Glycosylphosphatidylinositol biosynthesis defect 2
- Hypophosphatasia with mental retardation syndrome 1 (HPMRS1)
- Mabry Syndrome
- PIGV deficiency
Clinical Presentation
Individuals with PIGV-CDG typically develop signs and symptoms during infancy. PIGV-CDG is primarily characterized by severe global developmental delay, dysmorphic features, and hyperphosphatasia. Symptoms of PIGV-CDG include1–6,10:
- Neurological– developmental delay in speech and language development, epileptic seizures, and low muscle tone (hypotonia).
- Dysmorphic features – short finger bones (brachytelephalangy), wide set eyes, short nose with broad nasal bridge and tip, cleft palate, and tented upper lip.
Other symptoms include Hirschsprung disease (a congenital condition affecting the large intestine), vesicoureteral, renal anomalies, anorectal malformations, and heart defects.
Biochemical Abnormalities
Biochemical abnormalities observed in individuals with PIGV-CDG include elevated alkaline phosphatase levels. Alkaline phosphatase is a GPI-anchor protein that is normally found on the cell surface but may be secreted as a soluble protein in PIG deficiencies10,18,19.
Classification
PIGV-CDG is classified as a disorder of GPI-anchor biosynthesis.
Diagnosis
GPI-related CDG should be considered in individuals presenting with early onset severe seizure disorders and dysmorphic facial features, even if transferrin and total N-glycan analysis are normal. As currently available screening tests for CDG will not reliably detect PIGV-CDG, diagnosis is typically achieved through genetic testing, either as part of an epilepsy panel or whole exome sequencing. PIGV-CDG may also be diagnosed by analyzing surface GPI-anchor proteins on blood cells and fibroblast cells by flow cytometry7.
GPI-Anchored Protein Flow Cytometry
Individuals with PIGV-CDG lack GPI-anchored proteins on the surface of their granulocytes and in fibroblast cells7.
Biomarkers
There are currently no known biomarkers specific to PIGV-CDG.
Prognosis
Prognosis of PIGV-CDG may vary depending on severity of an individual’s symptoms. The broad clinical spectrum may result in premature death in some patients10. Patients have been reported with PIGV-CDG ranging from newborn to 19 years old4.
Management
Management of symptoms may include combinations of physical therapy, occupational therapy, and palliative measures. In some patients, seizures are treatable with anti-epileptic medication10.
Therapies
There are currently no treatment options available for PIGV-CDG.
Research Models
Mouse research models are available to study PIGV-CDG.
Mouse (M. musculus)
Homozygous PigvA341E mouse
Homozygous PigvAA341E is CRISPR-Cas9 induced mutation of the most prevalent hypomorphic missense mutation in European patients c.1022C>A (p.A341E). Mutant mice presented with motor coordination deficits, cognitive impairment, altered behaviour and sleep patterns, and increased seizure susceptibility20.
Pigv deletion embryonic stem cell line
Pigvtm1(KOMP)Mbp is a reporter-tagged deletion embryonic stem cell line. Phenotype data is not available (IMPC).
Clinical Studies
Active
Clinical and Basic Investigations into Congenital Disorders of Glycosylation (NCT04199000)
The Frontiers in Congenital Disorder of Glycosylation Disorders Consortium (FCDGC) is conducting a 5-year natural history study on all CDG types, including PIGV-CDG. The purpose of this study is to define the natural history and clinical symptoms of CDG, develop new diagnostic techniques, identify clinical biomarkers that can be used in future clinical trials and evaluate whether dietary treatments improve clinical symptoms and quality of life.
Organizations
GPI-anchor CDG Community Facebook Group
Mabry Syndrome Family Support Facebook Group
Publications
PIGV-CDG Scientific Articles on PubMed
Additional Resources
Kinoshita Lab (GPI anchor pathway researchers)
References
1. Krawitz, P. M. et al. Identity-by-descent filtering of exome sequence data identifies PIGV mutations in hyperphosphatasia mental retardation syndrome. Nat Genet, 42, 827-829 (2010).
2. Reynolds, K. K., Juusola, J., Rice, G. M. & Giampietro, P. F. Prenatal presentation of Mabry syndrome with congenital diaphragmatic hernia and phenotypic overlap with Fryns syndrome. Am J Med Genet A 173, 2776–2781 (2017).
3. Xue, J., Li, H., Zhang, Y. & Yang, Z. Clinical and genetic analysis of two Chinese infants with Mabry syndrome. Brain Dev 38, 807–818 (2016).
4. Horn, D. et al. Delineation of PIGV mutation spectrum and associated phenotypes in hyperphosphatasia with mental retardation syndrome. Eur J Hum Genet 22, 762-767 (2014)
5. Horn, D., Krawitz, P., Mannhardt, A., Korenke, G. C. & Meinecke, P. Hyperphosphatasia-Mental Retardation Syndrome Due to PIGV Mutations: Expanded Clinical Spectrum. Am J Med Genet A 155A, 1917-1922 (2011).
6. Thompson, M. D. et al. Phenotypic variability in hyperphosphatasia with seizures and neurologic deficit (Mabry syndrome). Am J Med Genet A 158A, 553-558 (2012).
7. Knaus, A. et al. Characterization of glycosylphosphatidylinositol biosynthesis defects by clinical features, flow cytometry, and automated image analysis. Genome Med 10, (2018).
8. Ji, Y. K. et al. PIG-V involved in transferring the second mannose in glycosylphosphatidylinositol. Journal of Biological Chemistry 280, 9489–9497 (2005).
9. PIGV Gene - GeneCards | PIGV Protein | PIGV Antibody. https://www.genecards.org/cgi-bin/carddisp.pl?gene=PIGV#summaries.
10. OMIM Entry - # 239300 - HYPERPHOSPHATASIA WITH MENTAL RETARDATION SYNDROME 1; HPMRS1. https://www.omim.org/entry/239300.
11. Kinoshita, T. Biosynthesis and biology of mammalian GPI-anchored proteins. Open Biology 10, (2020).
12. Kinoshita, T. Glycosylphosphatidylinositol (GPI) anchors: Biochemistry and cell biology: Introduction to a thematic review series. Journal of Lipid Research 57, (2016).
13. Liu, Y. S. & Fujita, M. Mammalian GPI-anchor modifications and the enzymes involved. Biochemical Society Transactions 48, (2020).
14. Englund, P. T. The Structure and Biosynthesis of Glycosyl Phosphatidylinositol Protein Anchors. Annual Review of Biochemistry 62, (1993).
15. Maeda, Y. & Kinoshita, T. Structural remodeling, trafficking and functions of glycosylphosphatidylinositol-anchored proteins. Progress in Lipid Research 50, (2011).
16. Fujita, M. & Kinoshita, T. Structural remodeling of GPI anchors during biosynthesis and after attachment to proteins. FEBS Letters 584, (2010).
17. Yuan, X. et al. A hypomorphic PIGA gene mutation causes severe defects in neuron development and susceptibility to complement-mediated toxicity in a human iPSC model. PLoS ONE 12, (2017).
18. Murakami, Y. et al. Mechanism for Release of Alkaline Phosphatase Caused by Glycosylphosphatidylinositol Deficiency in Patients with Hyperphosphatasia Mental Retardation Syndrome. Journal of Biological Chemistry 287, 6318–6325 (2012).
19. Murakami, Y. et al. Release of Alkaline Phosphatase Caused by PIGV Mutations In Patients with Hyperphosphatasia-Mental Retardation Syndrome (HPMR), a Recently Found Second Inherited GPI Anchor Deficiency. Blood 116, 2031–2031 (2010).
20. Rodríguez De Los Santos, M. et al. A CRISPR-Cas9-engineered mouse model for GPI-anchor deficiency mirrors human phenotypes and exhibits hippocampal synaptic dysfunctions. Proc Natl Acad Sci U S A 118, 1-11 (2021).
Show More