Lay Summary
PIGT-CDG is a rare inherited condition that affects several systems in the body. To date, 50 patients with PIGT-CDG have been reported in the medical literature. PIGT-CDG is also commonly referred to as PIGT deficiency or Multiple Congenital Anomalies-Hypotonia-Seizures syndrome type 3 (MCAHS3). PIGT-CDG is classified as a disorder of GPI anchor biosynthesis. It is caused when an individual has mutations in both copies of their PIGT gene, which provides instructions for making a protein that participates in attaching GPI anchors to proteins. GPI anchors are molecules that “anchor” certain proteins to the cell surface. The PIGT protein is involved in stabilizing the cell machinery that attaches GPI anchors to proteins. Mutations in the PIGT gene cause defects in GPI-anchored proteins, making them unstable or unable to attach to the cell surface. Symptoms of PIGT-CDG begin in infancy and are primarily characterized by intellectual disability, reductions in brain size, epilepsy, reduced muscle mass, and vision problems. Skeletal and joint problems, as well as facial abnormalities, may also be present in some individuals with this disorder. Some individuals diagnosed with PIGT-CDG may have a lower life expectancy due to complications. PIGT-CDG is usually diagnosed through genetic testing, however testing for the presence of GPI-anchored proteins on certain blood cells can also help screen for PIGT-CDG. With the exception of ketogenic diets, which may reduce seizures, there are currently no approved treatments for PIGT-CDG. Present treatment is focused on the management of specific symptoms and preventing complications.
Overview
Phosphatidylinositol glycan class T congenital disorder of glycosylation (PIGT-CDG) is a rare autosomal recessive genetic disorder. The first reported case of PIGT-CDG was in 2013, and there are now 50 confirmed cases to date1–11. PIGT-CDG is also commonly referred to as PIGT deficiency or Multiple Congenital Anomalies-Hypotonia-Seizures syndrome type 3 (MCAHS3).
The PIGT gene encodes a subunit of the enzyme complex that catalyzes the attachment of GPI anchors to proteins during GPI-anchored protein biosynthesis. GPI anchors are an important mechanism of attachment for proteins to the cell surface, and these proteins are known as GPI-anchored proteins. Mutations in PIGT result in defects in GPI-anchored proteins, which are critical to the development and function of many organs, especially the nervous system12. Combined somatic and germline mutations in PIGT alleles have been identified in patients with paroxysmal nocturnal hemoglobinuria (PNH), a rare acquired blood disorder13.
Symptoms begin at infancy and the characteristic presentation includes intellectual disability, developmental delay, seizures, epilepsy, facial abnormalities as well as decreased muscle tone2,14. Although a definitive diagnosis can only be achieved through genetic sequencing, analyzing blood cells for an absence of GPI-anchored proteins can help diagnose disorders of GPI anchor biosynthesis15. No treatment is currently available for PIGT-CDG, and treatment consists of management of symptoms while preventing complications.
Synonyms
- Congenital Disorder of Glycosylation due to PIGT deficiency
- Multiple Congenital Anomalies-hypotonia-seizures syndrome type 3
- MCAHS3
- GPI biosynthesis defect type 7
Inheritance
PIGT-CDG is an autosomal recessive disorder, meaning an affected individual inherits one defective copy of the gene from each asymptomatic parent.
Gene Function
The PIGT gene encodes PIG-T, a subunit of the 5-protein glycosylphosphatidylinositol (GPI) transamidase complex that catalyzes the attachment of a GPI anchor to a protein in the ER16. PIG-T helps stabilize the GPI transamidase complex during GPI anchor attachment to proteins16.
GPI-Anchored Protein Biosynthesis
GPI-anchored protein biosynthesis is one of the major glycosylation pathways that attach glycans to lipid molecules within cells. Many proteins, called GPI-anchored proteins, are attached to the cell surface by GPI anchors.
The GPI core structure consists of phosphatidylinositol (PI), glucosamine (GlcN), three mannose sugars (Man3), and three phosphoethanolamine (EtN-P) connected to each other in that sequence (Figure 1). GPI-anchored proteins are attached to the GPI by forming a bond between the EtN-P group of the GPI core and the C-terminus of newly synthesized proteins in the ER lumen17.
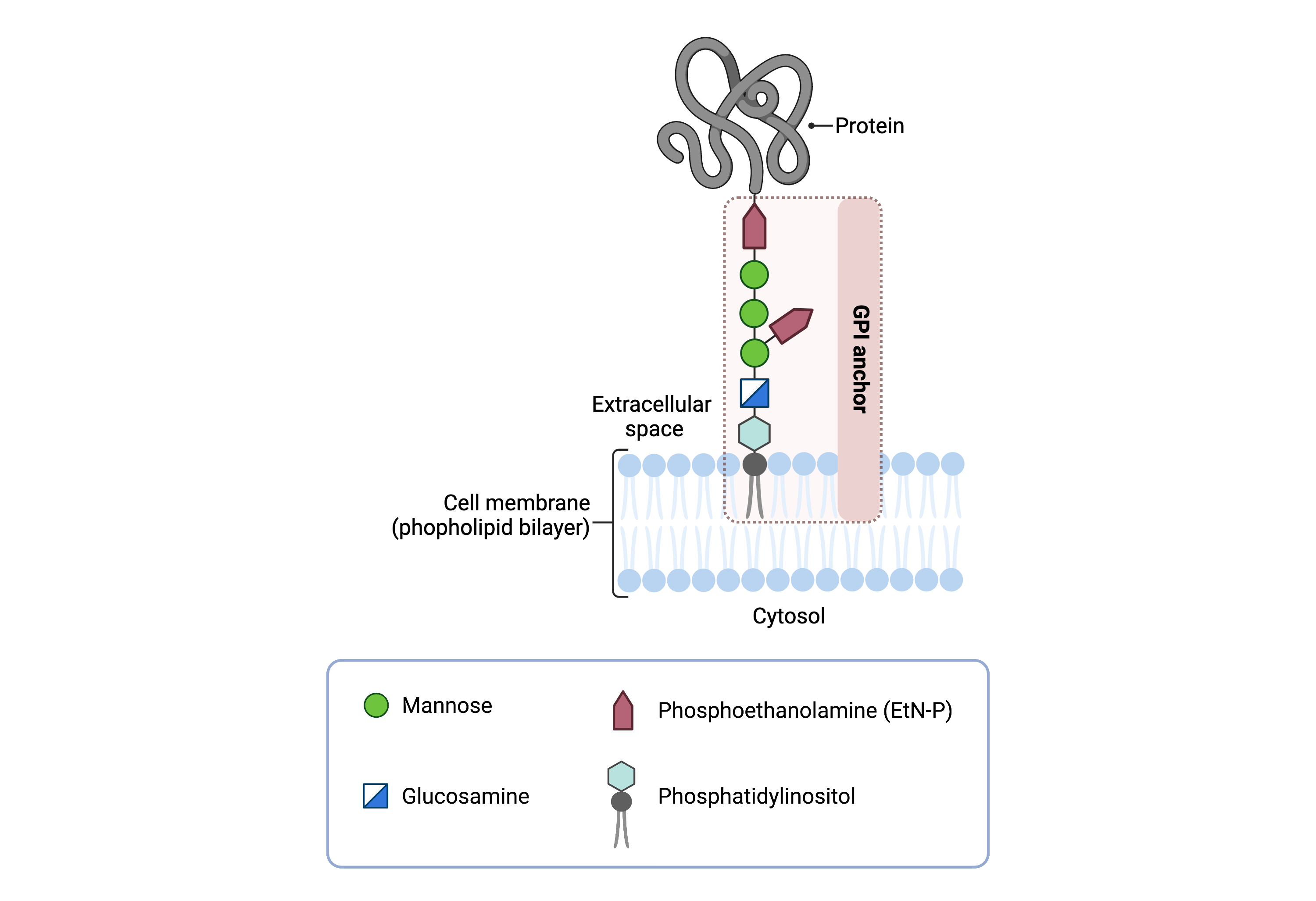
Figure 1. Overview of GPI-anchored protein structure.
The GPI anchor is added to proteins, generating GPI-anchored proteins. The GPI anchor section lodges into the membrane, attaching the protein to the membrane. The GPI anchor involves phosphatidylinositol, glucosamine, mannose and phosphoethanolamine.
- GPI anchor synthesis
- Protein attachment
- Lipid/glycan remodelling and protein transport
GPI anchor synthesis and protein attachment is largely carried out by a series of enzymes encoded by the PIG genes, while enzymes encoded by the PGAP genes facilitate remodelling of the GPI anchored protein.
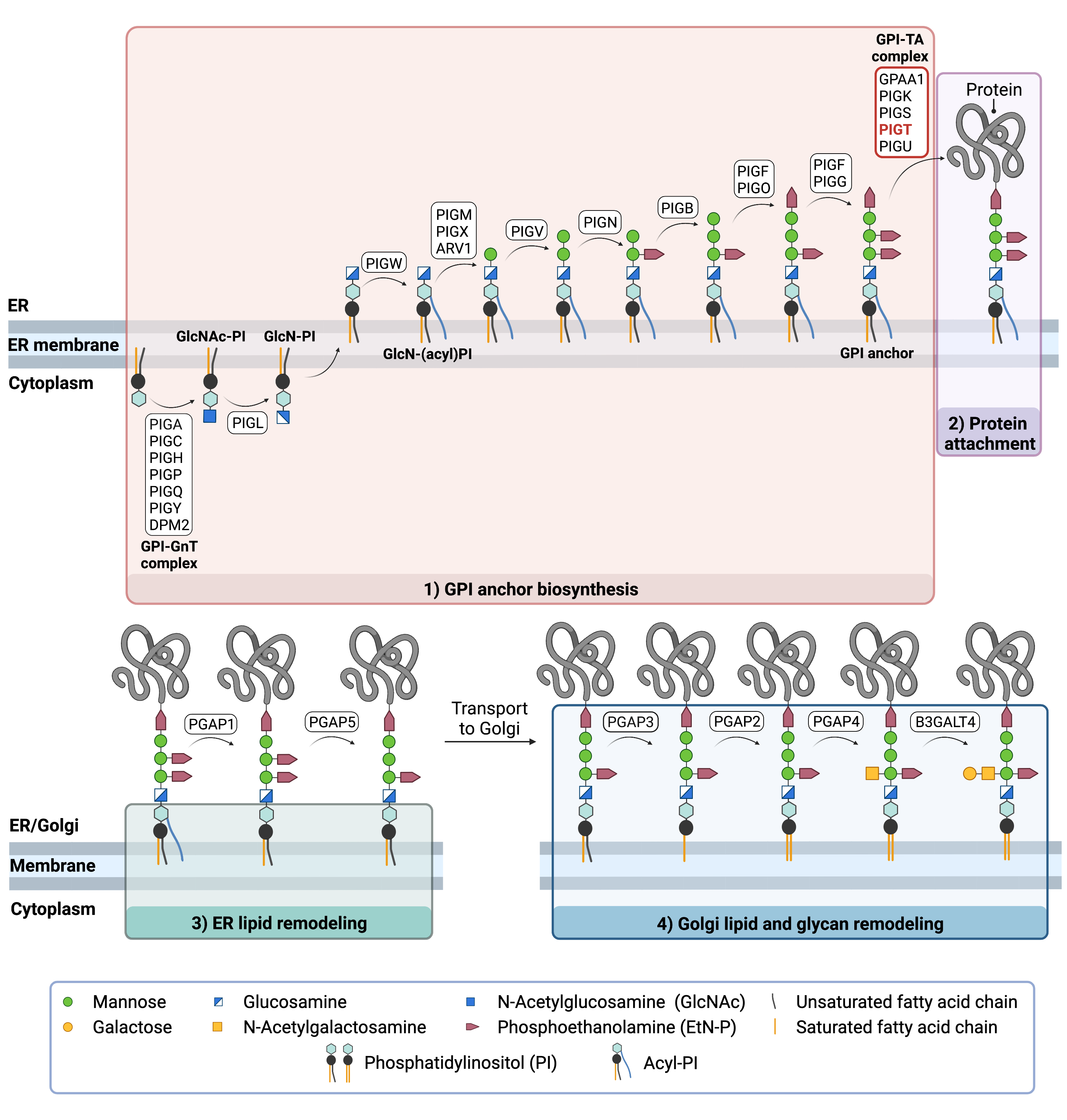
Figure 2. Overview of GPI-anchored protein biosynthesis.
GPI-anchored protein biosynthesis relies on a series of enzymatic reactions. First, the GPI anchor core is built on the ER membrane, where enzymes modify the lipid and glycan portions, generating
PI-GlcN-Man3-EtN-P. The protein is then attached to the GPI anchor in the ER, before further modifications are made to the lipid and glycan portions in the ER and the Golgi.
GPI ANCHOR SYNTHESIS
The first stage of GPI anchor biosynthesis involves the stepwise construction of the GPI anchor. N-acetylglucosamine (GlcNAc) is added to the lipid phosphatidylinositol (PI), generating GlcNAc-PI. The transfer of GlcNAc is catalyzed by GPI GlcNAc transferase (GPI-GnT). Additional enzymes then further modify the GPI anchor structure, generating the GPI anchor core (PI-GlcN-Man3-EtN-P)19,21.
PROTEIN ATTACHMENT
The GPI anchor core is transferred en bloc to a protein by GPI transamidase (GPI-TA). GPI transamidase is a large enzyme complex comprised of 5 proteins: PIG-K, GAA1, PIG-U, PIG-T, and PIG-S. PIG-T is essential for the formation of the GPI transamidase complex and helps stabilize the catalytic subunits PIG-K and GAAI proteins22,23. Upon recognition of the signal sequence, GPI transaminase catalyzes the simultaneous cleavage of the signal sequence and attachment of the GPI anchor to the newly synthesized protein18.
LIPID/GLYCAN REMODELLING AND PROTEIN TRANSPORT
After the protein has been attached to the GPI anchor, both the glycan and lipid portion of the anchor undergo modifications (referred to as remodeling) in the ER and Golgi by post-GPI attachment to protein (PGAP) enzymes or the glycosyltransferase B3GALT4. The complete GPI-anchored protein is then transported to the plasma membrane where it associates with other GPI-anchored proteins in lipid rafts24.
Disease Mechanism
Mutations in the PIGT gene lead to a reduction in functional GPI-anchored proteins, as PIGT encodes an essential subunit of the GPI transamidase complex required for attachment of GPI anchors to proteins with a GPI attachment signal2. GPI anchor proteins have several critical functions in the cell such as embryogenesis, immune response, and neurogenesis22,23.
Mutations
The PIGT gene is located on chromosome 20 (20q13.12)14. To date, 13 variants in the PIGT gene have been reported, including missense and splice-site variants2. The missense variant c.1582G>A (p.Val528Met) may be associated with a milder phenotype as patients with this mutation present with treatable epilepsy2,10,25. However, broader conclusions cannot be made with the limited number of patients reported so far.
Signs & Symptoms
Clinical Presentation
Individuals with PIGT-CDG typically develop signs and symptoms during infancy. PIGT-CDG is primarily characterized by severe to profound global developmental delay and refractory epilepsy. Symptoms of PIGT-CDG include2,3,5,7,8,25:
- Neurological – global developmental delay, intellectual disability, low muscle tone (hypotonia), epileptic seizures, and underdevelopment of the cerebellum (cerebellar hypoplasia)
- Dysmorphic features – facial bone abnormalities (high forehead, full cheeks, open mouth, sparse eyebrows, eye lashes and scalp hair, and short nose), abnormal curvature of the spine (scoliosis), and bone defects resulting in fracture (congenital fractures)
- Ophthalmological – involuntary side-to-side movement of eyes (nystagmus), crossed eyes (strabismus), and vision problems originating from the brain (cortical visual impairment)
Less common symptoms of PIGT-CDG include hearing loss, intestinal abnormalities, and heart disease, namely congenital heart disease and atrial septal defects.
Biochemical Presentation
Biochemical abnormalities observed in some individuals with PIGT-CDG include reduced levels of alkaline phosphatase in the blood, increased calcium concentrations due to bone problems, and low levels of parathyroid hormone15.
Classification
PIGT-CDG is classified as a disorder of GPI anchor biosynthesis.
Diagnosis
GPI-related CDG should be considered in individuals presenting with early onset severe seizure disorders and dysmorphic facial features, even if transferrin and total N-glycan analysis are normal1. As currently available screening tests for CDG will not reliably detect PIGT-CDG, diagnosis is typically achieved through genetic testing, either as part of an epilepsy panel or whole exome sequencing. PIGT-CDG may also be primarily screened for by analyzing surface GPI-anchored proteins on blood cells by flow cytometry15.
GPI-Anchored Protein Flow Cytometry
Individuals with PIGT-CDG lack GPI-anchored proteins on the surface of their granulocytes, a type of white blood cell.
Biomarkers
There are currently no known biomarkers specific to PIGT-CDG.
Prognosis
Prognosis of PIGT-CDG may vary depending on severity of an individual’s symptoms. The broad clinical spectrum may result in premature death in some patients, as a result of respiratory or cardiac complications. Death has been reported as early as 6–15 months of age, while the oldest described patient in the literature is almost 30 years old2,15.
Management
Management of symptoms may include combinations of physical therapy, occupational therapy, and palliative measures. In some patients, seizures are treatable with anti-epileptic medication2,25,26.
Therapies
There are currently no treatment options available for PIGT-CDG.
Pyridoxine, a form of vitamin B6, has been investigated as a treatment for one PIGT-CDG patient with treatment-resistant epilepsy but was found to be ineffective27. Pyridoxine was investigated as a treatment for GPI disorders because it is thought that there may be a reduced level of intracellular pyridoxine, specifically in the brain, due to disrupted GPI-anchored protein function27.
Research Models
Research models available to study PIGT-CDG include zebrafish and mouse models.
Fish (D. rerio)
Morpholino-mediated knockdown of the PIGT orthologue (pigt) in zebrafish results in defects in development such as gastrulation problems within the embryo, shortened body axes, as well as longer somites, and broad and kinked notochords, which are components of the zebrafish embryo3,14.
Mouse (M. musculus)
Pigt-/- constitutive knockout mice
Intragenic deletion of Pigt has been engineered in the mutant mice strain B6N.129S5-Pigttm1Lex, which has exons 1–3 targeted by homologous recombination. Homozygosity results in prenatal lethality, while heterozygous mice have defects in bone metabolism28,29.
Clinical Studies
Active
Clinical and Basic Investigations into Congenital Disorders of Glycosylation (NCT04199000)
The Frontiers in Congenital Disorder of Glycosylation Disorders Consortium (FCDGC) is conducting a 5-year natural history study on all CDG types, including PIGT-CDG. The purpose of this study is to define the natural history and clinical symptoms of CDG, develop new diagnostic techniques, identify clinical biomarkers that can be used in future clinical trials and evaluate whether dietary treatments improve clinical symptoms and quality of life.
Organizations
GPI-anchor CDG Community Facebook Group
PIGT-CDG Support Facebook Group
Publications
PIGT-CDG Scientific Articles on PubMed
Additional Resources
Kinoshita Lab (GPI anchor pathway researchers)
OMIM
IEMbase
Orphanet
Genetic Testing Registry
ClinVar
NIH
GeneCards
UniProt
References
- Lam, C. et al. Expanding the clinical and molecular characteristics of PIGT-CDG, a disorder of glycosylphosphatidylinositol anchors. Molecular Genetics and Metabolism 115, 128–140 (2015).
- Bayat, A. et al. PIGT-CDG, a disorder of the glycosylphosphatidylinositol anchor: description of 13 novel patients and expansion of the clinical characteristics. Genet Med 21, 2216–2223 (2019).
- Kvarnung, M. et al. A novel intellectual disability syndrome caused by GPI anchor deficiency due to homozygous mutations in PIGT. J Med Genet 50, 521–528 (2013).
- Nakashima, M. et al. Novel compound heterozygous PIGT mutations caused multiple congenital anomalies-hypotonia-seizures syndrome 3. Neurogenetics 15, 193–200 (2014).
- Skauli, N. et al. Novel PIGT Variant in Two Brothers: Expansion of the Multiple Congenital Anomalies-Hypotonia Seizures Syndrome 3 Phenotype. Genes (Basel) 7, (2016).
- Knaus, A. et al. Characterization of glycosylphosphatidylinositol biosynthesis defects by clinical features, flow cytometry, and automated image analysis. Genome Med 10, (2018).
- Kohashi, K. et al. Epileptic apnea in a patient with inherited glycosylphosphatidylinositol anchor deficiency and PIGT mutations. Brain Dev 40, 53–57 (2018).
- Yang, L. et al. Homozygous PIGT Mutation Lead to Multiple Congenital Anomalies-Hypotonia Seizures Syndrome 3. Front Genet 9, (2018).
- Mason, S. et al. Case report of a child bearing a novel deleterious splicing variant in PIGT. Medicine 98, e14524 (2019).
- Jezela-Stanek, A. et al. Evidence of the milder phenotypic spectrum of c.1582G>A PIGT variant: Delineation based on seven novel Polish patients. Clin Genet 98, 468–476 (2020).
- Jiao, X. et al. Analyzing clinical and genetic characteristics of a cohort with multiple congenital anomalies-hypotonia-seizures syndrome (MCAHS). Orphanet J Rare Dis 15, (2020).
- Paulick, M. G. & Bertozzi, C. R. The Glycosylphosphatidylinositol Anchor: A Complex Membrane-Anchoring Structure for Proteins. Biochemistry 47, 6991 (2008).
- Murakami, Y. et al. Paroxysmal Nocturnal Hemoglobinuria Caused By Pigt Mutations; Atypical PNH. Blood 128, 2450–2450 (2016).
- OMIM Entry - * 610272 - PHOSPHATIDYLINOSITOL GLYCAN ANCHOR BIOSYNTHESIS CLASS T PROTEIN; PIGT. https://www.omim.org/entry/610272.
- PIGT-congenital disorder of glycosylation (PIGT-CDG, also known as phosphatidylinositol-glycan class T protein deficiency or Multiple Congenital Anomalies-Hypotonia-Seizures Syndrome 3) | Rare Diseases Clinical Research Network. https://www.rarediseasesnetwork.org/fcdgc/PIGT.
- Kawaguchi, K., Sato, T., Kondo, S., Yamamoto-Hino, M. & Goto, S. Stability of the transamidase complex catalyzing GPI anchoring of proteins. Biochemical and Biophysical Research Communications 512, 584–590 (2019).
- Kinoshita, T. Biosynthesis and biology of mammalian GPI-anchored proteins. Open Biology 10, 190290 (2020).
- Kinoshita, T. Glycosylphosphatidylinositol (GPI) anchors: Biochemistry and cell biology: Introduction to a thematic review series. Journal of Lipid Research vol. 57 (2016).
- Liu, Y. S. & Fujita, M. Mammalian GPI-anchor modifications and the enzymes involved. Biochemical Society Transactions vol. 48 (2020).
- Englund, P. T. The Structure and Biosynthesis of Glycosyl Phosphatidylinositol Protein Anchors. Annual Review of Biochemistry 62, (1993).
- Maeda, Y. & Kinoshita, T. Structural remodeling, trafficking and functions of glycosylphosphatidylinositol-anchored proteins. Progress in Lipid Research vol. 50 (2011).
- Ohishi, K., Nagamune, K., Maeda, Y. & Kinoshita, T. Two Subunits of Glycosylphosphatidylinositol Transamidase, GPI8 and PIG-T, Form a Functionally Important Intermolecular Disulfide Bridge. Journal of Biological Chemistry 278, 13959–13967 (2003).
- Ohishi, K. PIG-S and PIG-T, essential for GPI anchor attachment to proteins, form a complex with GAA1 and GPI8. The EMBO Journal 20, 4088–4098 (2001).
- Fujita, M. & Kinoshita, T. Structural remodeling of GPI anchors during biosynthesis and after attachment to proteins.FEBS Letters vol. 584 (2010).
- Pagnamenta, A. T. et al. Analysis of exome data for 4293 trios suggests GPI-anchor biogenesis defects are a rare cause of developmental disorders. European Journal of Human Genetics 25, 669–679 (2017).
- PIGT-congenital disorder of glycosylation (PIGT-CDG, also known as phosphatidylinositol-glycan class T protein deficiency or Multiple Congenital Anomalies-Hypotonia-Seizures Syndrome 3) | Rare Diseases Clinical Research Network. https://www.rarediseasesnetwork.org/index.php/fcdgc/PIGT.
- Bayat, A. et al. Pyridoxine or pyridoxal-5-phosphate treatment for seizures in glycosylphosphatidylinositol deficiency: A cohort study. Developmental Medicine & Child Neurology (2022) doi:10.1111/DMCN.15142.
- MMRRC:032522-UCD. https://www.mmrrc.org/catalog/sds.php?mmrrc_id=32522.
- Tang, T. et al. A mouse knockout library for secreted and transmembrane proteins. Nature Biotechnology 28, 749–755 (2010).
