Lay Summary
Overview
α-1,2-mannosyltransferase congenital disorder of glycosylation (ALG9-CDG) is a rare autosomal recessive genetic disorder. The first reported case of ALG9-CDG was in 20043 and there are 171–7 reported cases in the literature. The ALG9 (Asparagine-linked glycosylation 9) gene encodes an enzyme responsible for adding the seventh of nine mannose residues during synthesis of the lipid linked oligosaccharide (LLO) in the lumen of the endoplasmic reticulum (ER)1. LLO synthesis is a precursor step to N-glycosylation. Deficiency of the ALG9 enzyme results in the incomplete assembly of the LLO, leading to insufficient N-glycosylation of glycoproteins2.
Symptoms may begin prenatally, presenting as skeletal dysplasia. In infancy, the characteristic presentation of AL9-CDG includes abnormal features, global developmental disability, epilepsy, and gastrointestinal problems4. Mutations in ALG9 have also been associated with Gillessen-Kaesbach-Nishimura syndrome, a rare, severe syndrome characterized by skeletal dysplasia, polycystic kidneys, and other malformations5. A diagnosis can be determined through transferrin analysis and LLO analysis, although a definitive diagnosis can only be achieved through molecular genetic testing. There are currently no approved treatments for ALG9-CDG1,4.
Synonyms
- CDG-IL
- Congenital disorder of glycosylation type 1L
- Mannosyltransferase 7-9 deficiency
Inheritance
ALG9-CDG is an autosomal recessive disorder, meaning an affected individual inherits one defective copy of the gene from each asymptomatic parent4.
Gene Function
ALG9 encodes a mannosyltransferase enzyme, α-1,2-mannosyltransferase (ALG9). Mannosyltransferases are enzymes that enable the transfer of mannose during glycosylation. ALG9 is located in the ER membrane, facing the lumen, where it has a role in the assembly of the LLO, a precursor for protein N-glycosylation1. The ALG9 enzyme transfers the seventh of nine mannose residues from dolichol-phosphate-mannose (Dol-P-mannose) in the ER to the growing oligosaccharide prior to its attachment to a protein1.
LLO synthesis
N-glycosylation is the process by which an oligosaccharide is attached to the nitrogen atom of asparagine residues on proteins. N-glycosylation is initiated in the ER and begins with the synthesis of the LLO8. The LLO is comprised of a 14-sugar oligosaccharide attached to the lipid carrier dolichol pyrophosphate (Dol-PP). The 14-sugar glycan is comprised of 2 N-acetylglucosamine, 9 mannose, and 3 glucose residues. Once assembled, the oligosaccharide is transferred en bloc to proteins and undergoes further processing in the ER and Golgi8. Once the oligosaccharide is attached to a protein, it is referred to as an N-glycan.
LLO synthesis is carried out by a series of enzymes encoded by the DPAGT1 and ALG genes and can be divided into two phases: Phase I and Phase II 8 (Figure 1).
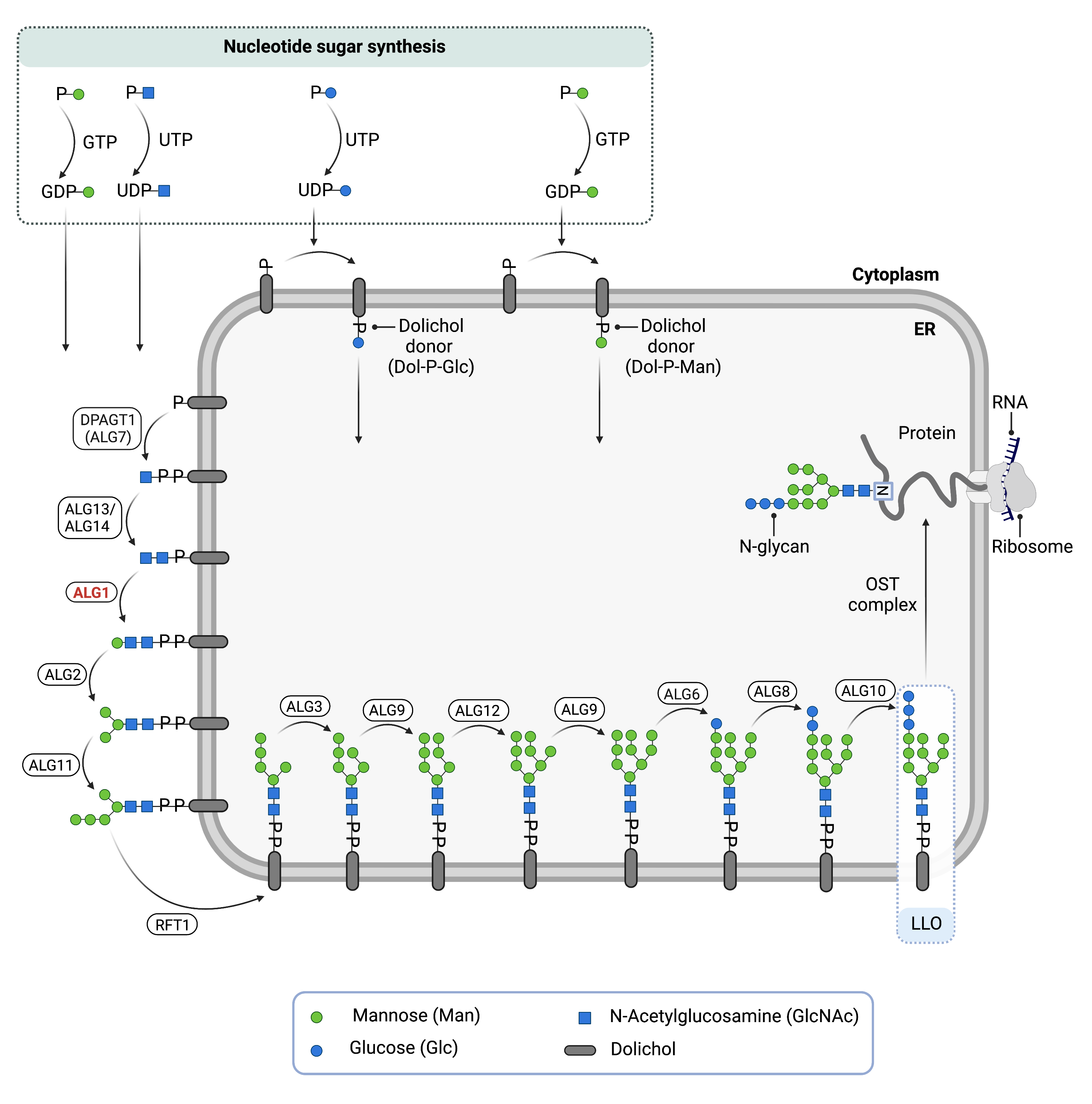
Figure 1. The Role of ALG9 in glycosylation.
ALG9 is an enzyme (α-1,2-mannosyltransferase) involved in synthesizing the lipid-linked oligosaccharide (LLO) in N-glycosylation. ALG9 catalyzes the transfer of the seventh mannose residue from Dol-P-mannose onto the LLO in the endoplasmic reticulum lumen.
Phase I
Phase I of LLO synthesis takes place on the cytoplasmic side of the ER. It begins with the sequential attachment of two N-Acetylglucosamine (GlcNAc2) and five mannose (Man5) residues to Dol-PP by several ER enzymes. GlcNAc and mannose are transferred from nucleotide sugars UDP-GlcNAc and GDP-mannose, respectively. The intermediate structure, Dol-PP-Man5GlcNAc2 is translocated across the ER membrane into the lumen by the RFT1 enzyme,9
Phase II
Phase II of LLO synthesis takes place in the ER lumen. Once in the lumen, four mannose residues followed by three glucose (Glc3) residues are added to the intermediate structure to generate the complete LLO, Dol-PP-GlcNAc2Man9Glc3. Mannose and glucose residues are transferred from glycosyl donors Dol-P-mannose and Dol-P-glucose, which are formed on the cytoplasmic side of the ER and must also be flipped across the ER membrane8. ALG9 catalyzes the transfer of the seventh mannose from Dol-P-mannose onto the growing LLO.
Once assembled, the oligosaccharide is transferred en bloc from Dol-PP to asparagine residues of newly synthesized proteins via the enzyme oligosaccharyltransferase (OST), resulting in N-glycosylation of the protein8. The activity of OST is highly specific for the completely assembled 14-sugar oligosaccharide, Glc3Man9GlcNAc2.The mannose residue added to by ALG9 is important for proper folding and proofreading of the N-glycoprotein in the ER and Golgi.
Disease Mechanism
Mutations in the ALG9 gene lead to the production of an abnormal enzyme with reduced activity. In the absence of ALG9, the LLO is missing three mannose and three glucose residues and is therefore incompletely assembled. This results in reduced transfer efficiency of the oligosaccharide by OST, causing an accumulation of the incomplete LLO, Dol-PP-GlcNAc210 and hypoglycosylation of glycoproteins which lack N-glycans9.
Mutations
The ALG9 gene is located on chromosome 11 (11q23.1). Most ALG9-CDG patients reported in the literature have homozygous missense variants. Splicing variants appear to be associated with more severe phenotype resulting in prenatal death while missense mutations are associated with a less severe phenotype1.
Signs & Symptoms
Clinical Presentation
Individuals with ALG9-CDG develop signs and symptoms that may be detected prenatally and in infancy, as early as a few days old. ALG9-CDG is primarily characterized by cognitive impairment and global developmental delay, hypotonia, epileptic seizures, brain structure abnormalities, microcephaly and skeletal abnormalities. The characteristic clinical presentations of ALG9-CDG include7:
- Neurological – cognitive impairment and developmental delay, decreased muscle tone (hypotonia), seizures, smaller than average head at birth (microcephaly). Cerebral anomalies including delayed myelination, global cerebral and cerebellar atrophy.
- Dysmorphic features – Skeletal dysplasia, inverted nipples, facial dysmorphisms; prominent forehead, big mouth, depressed nasal bridge, low-set ears and increased distance between the eyes.
- Gastrointestinal – enlarged liver (hepatomegaly) and other intestinal problems may lead to failure to gain weight and slower than normal growth (failure to thrive).
Organ dysfunction involving the liver, heart, lung and the kidney is also characteristic in ALG9-CDG patients.
Less commonly reported symptoms in patients include hearing loss, West Syndrome and hypsarrhythmia (abnormal brain electrical activity).
Gillessen-Kaesbach-Nishimura syndrome
Individuals with severe form of ALG9-CDG, Gillessen-Kaesbach-Nishimura syndrome, may present with polycystic kidneys, liver fibrosis, congenital heart disease, and abnormalities of cartilage and bone growth (skeletal dysplasia)5.
Biochemical Abnormalities
There have been no reports of significant biochemical abnormalities in ALG9-CDG patients to date. This includes normal metabolic screen, plasma amino acids, urine organic acids, plasma acylcarnitine profile, very-long chain fatty acids, serum biotinidase, serum 7-dehydrocholesterol, urinary 3-methylglutaconic acid, sialic acid, uronic acid, and oligosaccharide electrophoresis1.
Classification
ALG9-CDG is classified as a disorder of N-linked protein glycosylation.
Under the former CDG classification system, ALG9-CDG is classified as a Type 1 CDG, which arise due to defects in the synthesis of oligosaccharides or their transfer to proteins.
Diagnosis
Although diagnosis of ALG9-CDG may be suspected based on presentation of symptoms and detailed patient history, direct molecular genetic testing is the only definitive diagnostic test. Screening in suspected patients begins with a blood test to analyze serum transferrin. LLO analysis in patient fibroblasts may also be carried out following transferrin screening1.
Transferrin Analysis
Individuals with ALG9-CDG show a type 1 pattern by transferrin isoelectric focusing (TIEF) or mass spec analysis of transferrin. Type 1 patterns are observed in CDG that arise due to defects in LLO (Figure) and are characterized by a decrease in tetrasialo-transferrin and an increase in di-sialo and a-sialo transferrin glycoforms1.
LLO Analysis
LLO analysis of patient fibroblasts show a significant increase of N-linked GlcNAc2Man4, GlcNAc2Man5 and GlcNAc2Man6, and an absence of GlcNAc2Man7, GlcNAc2Man8, and GlcNAc2Man9 resulting from ALG9 deficiency1.
Biomarkers
Abnormal N-glycan profiles
Absent Man3 glycans and high abundance of Man4 glycans is observed in ALG9-CDG patients8.
Prognosis
Prognosis of ALG9-CDG may vary depending on the severity of an individual’s symptoms. Prenatal death has been reported in 3 patients who presented with Gillessen-Kaesbach–Nishimura skeletal dysplasia4.
Management
Management of symptoms may include combinations of physical therapy, occupational therapy, speech therapy and palliative measures.
Therapies
There are currently no treatment options available for ALG9-CDG. Treatment is focused on management of symptoms and prevention of complications1.
Research Models
Several ALG9 research models have been generated including yeast, fly, and mouse models.
Yeast (S. cerevisiae)
Alg9 null mutants are sensitive to reducing agents and display under-glycosylation of secreted proteins. In large-scale studies, myo-inositol is required and mutants show an Opi-phenotype (overproductions and excretion of inositol in the absence of inositol and choline), as well as reduced desiccation resistant and innate thermotolerance (Yeast Genome).
Fly (D. melanogaster)
Drosophila gene Alg9 (ALG9, CG11851, FBgn0039293) is orthologous to ALG9. It is predicted to have mannosyltransferase activity and be involved in protein glycosylation. It is expressed in the adult drosophila head and is a potential model for human disease ALG9-CDG (FlyBase).
Mouse (M. Musculus)
Homozygous Alg9 -/- Knockout
Alg9em1(IMPC)J homozygous and heterozygous mutant mice have been generated with an exon deletion. Homozygous mice live from E12.5 to early adult and phenotypically present with embryonic lethality prior to tooth bud stage or preweaning lethality and complete penetrance. Heterozygous mutants live to early adulthood and phenotypically show an increased amount of total body fat (IMPC).
Alg9 conditional-ready floxed knockout ES cell line
Embryonic stem (ES) cell line Alg9tm1a(EUCOMM)Wtsi is a targeted knockout/null mutation of Alg9. A “conditional-ready” allele can be created by flp recombinase expression in mice carrying this allele, and cre expression results in a knockout mouse. If cre is expressed without flp expression, a reporter knockout mouse can be generated (MGI).
Alg9 knockout ES cell line
Embryonic Stem (ES) cell line Alg9tm1e(EUCOMM)Wtsi is a targeted knockout/null mutation of Alg9 (MGI).
Clinical Studies
Active
Clinical and Basic Investigations into Congenital Disorders of Glycosylation (NCT04199000)
The Frontiers in Congenital Disorder of Glycosylation Disorders Consortium (FCDGC) is conducting a 5-year natural history study on all CDG types, including ALG9-CDG. The purpose of this study is to define the natural history and clinical symptoms of CDGs, develop new diagnostic techniques, identify clinical biomarkers that can be used in future clinical trials and evaluate whether dietary treatments improve clinical symptoms and quality of life.
Publications
ALG9-CDG Scientific Articles on PubMed
Additional Resources
References
- Davis, K. et al. ALG9-CDG: New clinical case and review of the literature. Molecular Genetics and Metabolism Reports 13, (2017).
- Harada, Y., Ohkawa, Y., Kizuka, Y. & Taniguchi, N. Oligosaccharyltransferase: A gatekeeper of health and tumor progression. International Journal of Molecular Sciences 20, (2019).
- Frank, C. G. et al. Identification and Functional Analysis of a Defect in the Human ALG9 Gene: Definition of Congenital Disorder of Glycosylation Type IL. The American Journal of Human Genetics 75, (2004).
- AlSubhi, S. et al. Further Delineation of the ALG9-CDG Phenotype. JIMD Rep. 27, (2016)
- Tham, E. et al. A novel phenotype in N-glycosylation disorders: Gillessen-Kaesbach–Nishimura skeletal dysplasia due to pathogenic variants in ALG9. European Journal of Human Genetics 24, (2016).
- Weinstein, M. et al. CDG-IL: An infant with a novel mutation in theALG9 gene and additional phenotypic features. American Journal of Medical Genetics Part A 136A, (2005).
- Vleugels, W. et al. Quality control of glycoproteins bearing truncated glycans in an ALG9-defective (CDG-IL) patient. Glycobiology 19, (2009).
- Bieberich, E. Synthesis, Processing, and Function of N-glycans in N-glycoproteins. in (2014). d
- Stanley, P., Taniguchi, N. & Aebi, M. N-glycans. in Essentials of Glycobiology [Internet] (eds. Varki, A. et al.) (Cold Spring Harbor Laboratory Press, 2017).
