Lay Summary
ALG3-CDG, formerly known as CDG-Id, is a rare inherited condition that affects many parts of the body. To date, there are 43 patients reported in the literature. ALG3-CDG is classified as a disorder of N-linked protein glycosylation. ALG3-CDG is caused when an individual has mutations in both copies of their ALG3 gene, which encodes an enzyme that attaches the simple sugar mannose to growing sugar chains during N-glycosylation. Mutations in the ALG3 gene cause proteins to be under glycosylated, meaning these proteins have less sugars attached than healthy forms of the proteins. Symptoms of ALG3-CDG begin in infancy and are primarily characterized by neurological abnormalities, abnormal facial features, and eye problems. Biomarker analysis and several screening tests are available for ALG3-CDG, but a definitive diagnosis is achieved through genetic testing. Treatment is focused on the management of specific symptoms and preventing complications.
Overview
Alpha-1,3-mannosyltransferase congenital disorder of glycosylation (ALG3-CDG) is a rare autosomal recessive genetic disorder1,2. The first case of ALG3-CDG was reported in 19953, and there are 43 reported cases in the literature to date1,2,4–6. The ALG3 (asparagine-linked glycosylation 3) gene encodes an enzyme responsible for adding the sixth of nine mannose residues during the synthesis of the lipid-linked oligosaccharide (LLO) in the lumen of the endoplasmic reticulum (ER). LLO synthesis is a precursor step to N-glycosylation. Deficiency of theALG3 enzyme results in the incomplete assembly of the LLO, leading to insufficient N-glycosylation of glycoproteins1.
Symptoms begin in infancy and the characteristic presentations of ALG3-CDG include neurological symptoms, dysmorphic features, and ophthalmological problems1. A diagnosis can be determined through transferrin analysis, LLO analysis, and analysis of the potential biomarker Man0-4GlcNAc21. Definitive diagnosis can only be achieved through molecular genetic testing. There are currently no approved treatments for ALG3-CDG1.
Synonyms
- CDG-Id
- Asparagine-linked glycosylation 3
- Mannosyltransferase 6 deficiency
Inheritance
ALG3-CDG is inherited in an autosomal recessive fashion, where a mutation is inherited from each parent (asymptomatic carriers)1,2.
Gene Function
ALG3 encodes a mannosyltransferase enzyme, alpha-1,3-mannosyltransferase (ALG3). Mannosyltransferases are enzymes that enable the transfer of mannose during glycosylation. ALG3 is located in the endoplasmic ERmembrane, facing the ER lumen, where it has a role in the assembly of the LLO, a precursor for protein N-glycosylation1,2. The ALG3 enzyme transfers the sixth of nine mannose residues from dolichol-phosphate mannose (Dol-P-mannose) to the growing oligosaccharide chain prior to its attachment to a protein in the ER lumen.
LLO synthesis
N-glycosylation is the process by which an oligosaccharide is attached to the nitrogen atom of an asparagine residue on a protein. N-glycosylation is initiated in the ER and begins with the synthesis of an LLO7. The LLO is comprised of a 14-sugar oligosaccharide attached to the lipid carrier dolichol pyrophosphate (Dol-PP). The 14-sugar oligosaccharide has 2 N-acetylglucosamine, 9 mannose, and 3 glucose residues. Once assembled, the oligosaccharide is transferred “en bloc” to proteins, forming an N-glycosylated protein. Once the oligosaccharide is attached to a protein, it is referred to as an N-glycan. The N-glycan can undergo additional processing in the ER and Golgi7.
LLO synthesis is carried out by a series of enzymes encoded by the DPAGT1 and ALG genes and can be divided into two phases: Phase I and Phase II (Figure 1)7.
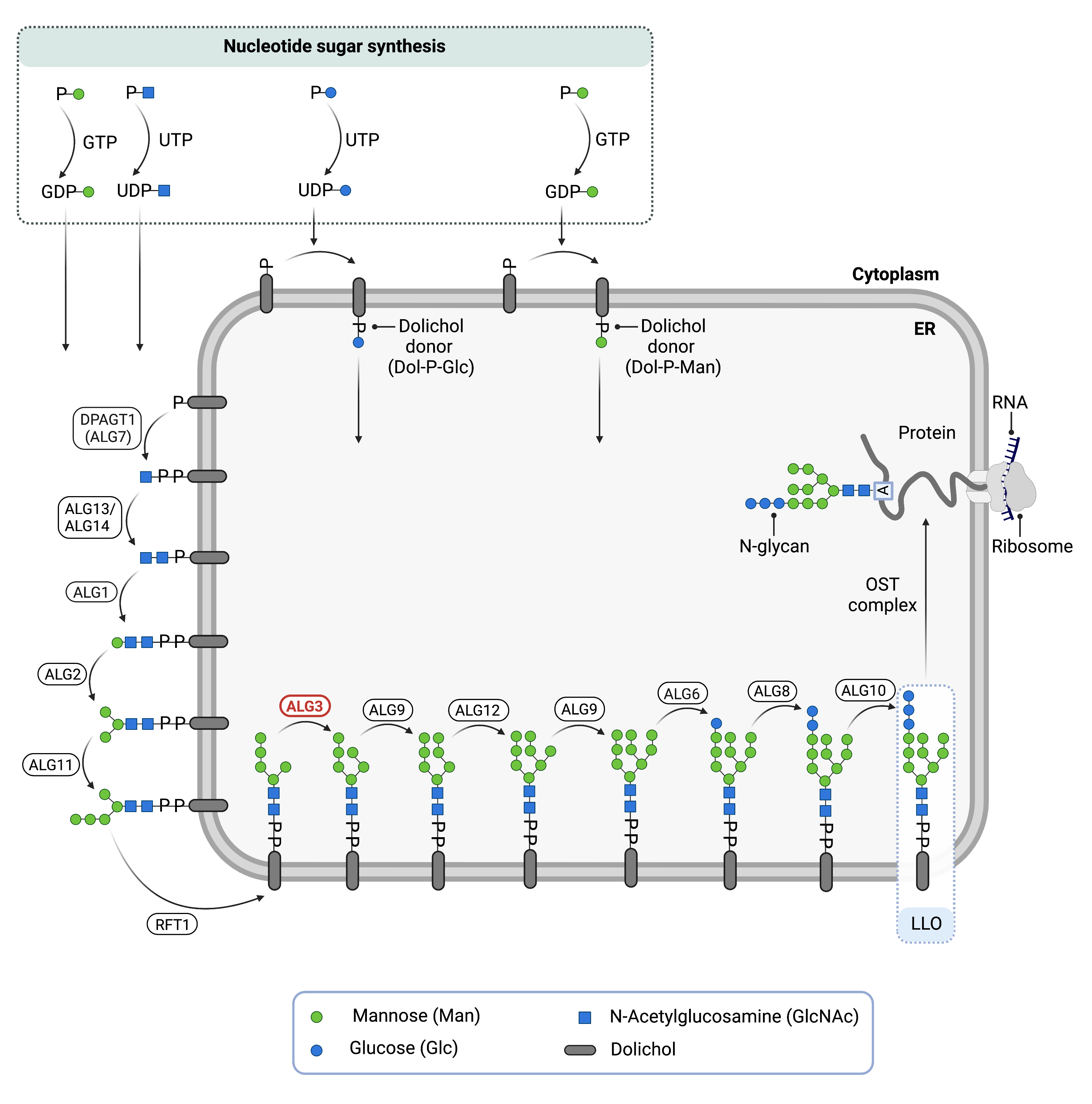
Figure 1. Role of ALG3 in glycosylation.
ALG3 is an enzyme (alpha-1,3-mannosyltransferase) involved in synthesising the lipid-linked oligosaccharide (LLO), which is needed for N-glycosylation. ALG3 adds a mannose residue to the LLO in the endoplasmic reticulum.
Phase I
Phase I of LLO synthesis takes place on the cytoplasmic side of the ER. It begins with the sequential attachment of two N-Acetylglucosamine (GlcNAc2) and five mannose (Man5) residues to Dol-PP by several ER enzymes. GlcNAc and Man are transferred from nucleotide sugars UDP-GlcNAc and GDP-mannse, respectively. The intermediate structure, Dol-PP-GlcNAc2Man5, is translocated from the cytoplasm into the ER lumen by the RFT1 enzyme7,9.
Phase II
Phase II of LLO synthesis takes place in the ER lumen. Once in the lumen, four mannose residues followed by three glucose (Glc3) residues are added to the intermediate structure, generating the complete N-glycan, Dol-PP-GlcNAc2Man9Glc3. Mannose and glucose are transferred from glycosyl donors Dol-P-mannose and Dol-P-glucose, which are formed on the cytoplasmic side of the ER and must also be flipped across the ER membrane7. ALG3 is responsible for adding the first of 9 mannose residues to the growing oligosaccharide in the ER lumen.
Once assembled, the oligosaccharide is transferred “en bloc” from Dol-PP to an asparagine residue of a newly synthesized protein via the enzyme oligosaccharyltransferase (OST), resulting in N-glycosylation of the protein7. The activity of OST is highly specific for the completely assembled 14-sugar oligosaccharide, Glc3Man9GlcNAc28. The mannose residue added to the oligosaccharide by ALG3 is also important for proper folding and proofreading of the glycoprotein in the ER and Golgi.
Disease Mechanism
Mutations in the ALG3 gene lead to ALG3 being unable to transfer mannose onto the growing oligosaccharide chain1. In the absence of ALG3, the LLO structure is incomplete. This results in a reduced transfer efficiency of the oligosaccharide by OST, and glycoproteins are hypoglycosylated. Additionally, there is an accumulation of the incomplete LLO structure: Dol-PP-GlcNAc2Man51.
Mutations
The ALG3 gene is located on chromosome 3 (3q27.1). There are 34 mutations reported for the ALG3 gene1,2. The majority of pathogenic mutations are missense, with small numbers of nonsense mutations and splice junction mutations1.
Signs & Symptoms
Clinical Presentation
Individuals with ALG3-CDG develop signs and symptoms during infancy and is primarily characterized by severe neurological disorder with dysmorphic features and visual impairment. ALG3-CDG clinical presentation may include a combination of2:
- Neurological – developmental delay, cerebral and cerebellar atrophy, agenesis of the corpus callosum, epilepsy, and a smaller than average head at birth (microcephaly)
- Musculoskeletal – bent wrist and hand joints (ulnar deviation), tightened or shortened joints (joint contractures), skeletal dysplasia, and scoliosis
- Dysmorphic features – down slanting palpebral fissures, abnormally wide set eyes (hypertelorism), high nasal bridge, anteriorly-facing nostrils, large low-set ears with abnormal pinnae, small lower jaw, thin lips, and inverted nipples
- Ophthalmological – visual impairment, poor eye fixation, strabismus, nystagmus, and compromised optic nerve atrophy/hypoplasia
- Gastrointestinal – intestinal problems and feeding difficulties may lead to failure to gain weight and slower than normal growth (failure to thrive)
Other clinical presentations of ALG3-CDG may include respiratory defects such as underdeveloped lungs, cardiac defects such as obstructive cardiomyopathy and dilated aorta, and urogenital abnormalities such as renal cysts, and swelling of a kidney due to urine buildup.
Biochemical Abnormalities
Biochemical abnormalities observed in individuals with ALG3-CDG include immunodeficiency, anemia, coagulation abnormalities, increased calcium levels in the kidneys, low levels of albumin in the blood (hypoalbuminemia), and endocrine abnormalities, such as hypothyroidism, central adrenal insufficiency, growth hormone deficiency, low blood sugar levels (hypoglycemia), and a decrease in plasma lipoprotein levels (hypolipidemia)1.
Classification
ALG3-CDG is classified as a disorder of N-linked protein glycosylation.
Under the former CDG classification system, ALG3-CDG is classified as a Type 1 CDG, which arise due to defects in the synthesis of oligosaccharides or their transfer to proteins.
Diagnosis
Although diagnosis of ALG3-CDG may be suspected based on the presentation of symptoms and a detailed patient history, direct molecular genetic testing is the only definitive diagnostic test. Screening in suspected patients begins with a blood test to analyze serum transferrin. LLO analysis in patient fibroblasts may also be carried out following transferrin analysis.
Transferrin Analysis
Individuals with ALG3-CDG show a type 1 pattern by transferrin isoelectric focusing (TIEF) or mass spec analysis of transferrin1. Type 1 patterns are observed in CDG that arise due to defects in LLO assembly and are characterized by a decrease in tetrasialo-transferrin and an increase in disialo- and asialo-transferrin isoforms5.
LLO Analysis
LLO analysis of ALG3-CDG patient fibroblasts show an increase in the intermediate LLO structure, Dol-PP-GlcNAc2Man51.
Biomarkers
Serum, plasma, and primary fibroblasts may be tested for Man0-4GlcNAc2 levels, which were shown to be increased in all tested individuals along with a reduction of Man9GlcNAc2. Man0-4GlcNAc2 may serve as a diagnostic biomarker for ALG3-CDG1.
Prognosis
Prognosis of ALG3-CDG may vary depending on the severity of an individual’s symptoms. Most ALG3-CDG patients reported in the literature to date are young and long-term prognosis information is unavailable. Severely affected patients may die in infancy or early childhood from complications of sepsis or seizures1. Less severely affected patients present with facial dysmorphism, developmental delay, seizures, and gastrointestinal symptoms6.
As of 2021, the oldest patient recorded in the medical literature has reached their thirties1.
Management
Management of symptoms may include combinations of physical therapy, occupational therapy, vision therapy, and palliative measures.
Therapies
There are currently no treatment options available for ALG3-CDG. Treatment is focused on the management of symptoms and prevention of complications.
Research Models
Several ALG3 models have been generated include yeast and mice, and there are several potential models in yeast, flies, worms, mice, and fish.
Yeast (S. cerevisiae)
Alg3-1 mutant yeast are defective in the biosynthesis of LLOs. Man5GlcNAc2-PP-Dol accumulates in alg3 cells and protein-bound carbohydrates are Endo H resistant10.
Fly (D. melanogaster)
Drosophila gene Alg3 (ALG3, CG4084, FBgn0011297) is orthologous to human ALG3. It is predicted to have mannosyltransferase activity and be involved in protein glycosylation. It is expressed in the adult drosophila head and is a potential model for human ALG3-CDG (FlyBase).
Worm (C. elegans)
Algn-3 is orthologous to human ALG3 and is predicted to have mannosyltransferase activity (WormBase).
Fish (D. rerio)
alg3 (ZDB-GENE-050522-334) is orthologous to human ALG3 and predicted to have mannosyltransferase and glucosyltransferase activity involved in protein glycosylation (ZFIN).
Mouse (M. musculus)
Alg3 -/- knockout mouse
C57BL/6NJ-Alg3em1(IMPC)J/Mmjax is an exon deletion mutant strain (MGI). Mice homozygous for the mutation live to early adulthood and phenotypically show preweaning lethality with complete penetrance. Heterozygous mice live to early adulthood and phenotypically show an increased heart rate and shortened RR interval (IMPC).
ALG3 conditional-ready floxed knockout embryonic stem cell line
Embryonic stem (ES) cell line Alg3tm1a(EUCOMM)Wtsi is a targeted knockout/null mutation of Alg3. A “conditional-ready” allele can be created by flp recombinase expression in mice carrying this allele, and cre expression results in a knockout mouse. If cre is expressed without flp expression, a reporter knockout mouse can be generated (MGI).
ALG3 knockout ES cell line
ES cell line Alg3tm1e(EUCOMM)Wtsi is a targeted knockout/null mutation of Alg3 (MGI).
Clinical Studies
Active
Clinical and Basic Investigations into Congenital Disorders of Glycosylation (NCT04199000)
The Frontiers in Congenital Disorder of Glycosylation Disorders Consortium (FCDGC) is conducting a 5-year natural history study on all CDG types, including ALG3-CDG. The purpose of this study is to define the natural history and clinical symptoms of CDG, develop new diagnostic techniques, identify clinical biomarkers that can be used in future clinical trials and evaluate whether dietary treatments improve clinical symptoms and quality of life.
Publications
ALG3-CDG Scientific Articles on PubMed
Additional Resources
OMIM
OrphaNet
NORD
GARD
Genetic Testing Registry
ClinVar
NIH
GeneCards
UniProt
References
- Alsharhan, H. et al. Expanding the phenotype, genotype and biochemical knowledge of ALG3‐CDG. Journal of Inherited Metabolic Disease 44, (2021).
- Farolfi, M. et al. ALG3-CDG: a patient with novel variants and review of the genetic and ophthalmic findings. BMC Ophthalmology 21, (2021).
- Stibler, H., Stephani, U. & Kutsch, U. Carbohydrate-Deficient Glycoprotein Syndrome - A Fourth Subtype. Neuropediatrics 26, (1995).
- Bian, Y. et al. ALG3-CDG: lethal phenotype and novel variants in Chinese siblings. Journal of Human Genetics 65, (2020).
- Denecke, J. et al. Congenital Disorder of Glycosylation Type Id: Clinical Phenotype, Molecular Analysis, Prenatal Diagnosis, and Glycosylation of Fetal Proteins. Pediatric Research 58, (2005).
- Riess, S. et al. ALG3-CDG (CDG-Id): Clinical, biochemical and molecular findings in two siblings. Molecular Genetics and Metabolism 110, (2013).
- Bieberich, E. Synthesis, Processing, and Function of N-glycans in N-glycoproteins. in (2014). doi:10.1007/978-1-4939-1154-7_3.
- Harada, Y., Ohkawa, Y., Kizuka, Y. & Taniguchi, N. Oligosaccharyltransferase: A gatekeeper of health and tumor progression. International Journal of Molecular Sciences vol. 20 (2019).
- Stanley, P., Taniguchi, N. & Aebi, M. N-glycans. in Essentials of Glycobiology [Internet] (eds. Varki, A. et al.) (Cold Spring Harbor Laboratory Press, 2017).
- Aebi, M., Gassenhuber, J., Domdey, H. & Heesen, S. te. Cloning and characterization of the ALG3 gene of Saccharomyces cerevisiae. Glycobiology 6, (1996).
